【干货】PDX与CDX
问:什么是肿瘤移植模型?
答:人的肿瘤移植到小鼠体内构建的人源化肿瘤模型
问:这种模型意义在哪?
答:筛药,为人民服务。(请为伟大的小鼠致敬!)
问:为啥要构建肿瘤模型,不能跳过直接上临床?不也可以筛药吗?
答:嗯,好主意,要不你先来好不好?
.......
问:什么是CDX , PDX?
答:急什么,认真听小编讲就是了。
CDX
人源肿瘤细胞系异种移植(cell derived xenograft, CDX),即将体外传代培养的肿瘤细胞接种至免疫缺陷小鼠,接种部位常为皮下,静脉或原位。
话说早在20世纪90年代早期,美国国立癌症研究所(National Cancer Institute, NCI)就 依据来源于9种不同类型肿瘤(脑,结肠癌,白血病,肺,黑素瘤、卵巢癌、肾癌、乳腺癌和前列腺癌) 的60位癌症患者肿瘤细胞系,引入了一种"disease-oriented"的药物筛选策略,简单来说,就是先用人肿瘤细胞系进行高通量体外药物筛选,再用CDX模型进行体内验证,【Cancer Cell Lines for Drug Discovery and Development 】
由于CDX模型细胞系容易获得,有大量已发表的文献关于其基因组学、细胞功能学及药效反应的数据可供参考,建模成本低等优势,【PDX模型与肿瘤个体化用药指导】在当时可以说是风靡各大实验室。
但是2016年,NCI却决定从其药筛系统中让已经使用了25年的NCI60细胞系“退休!
为什么会发生这种转变?!
原来,研究者逐渐发现人源肿瘤细胞系经长期体外培养后, 其肿瘤细胞生物学行为及基因谱表达水平、 肿瘤异质性都与原始肿瘤组织存在较大的差异, 从而在预测临床药效方面不甚理想。有研究表明, 经此模型鉴定筛选的药物仅约 1/3在二期临床试验中验证有效【Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials.】,【PDX 模型在肿瘤转化医学中的应用与发展】
不过嘛,长江后浪推前浪,退休了CDX,就会有更优秀的肿瘤模型崛起!这就是接下来小编要为大家重点介绍PDX啦~
PDX:
人源肿瘤组织来源移植瘤模型 (patientl derived xenograft ,PDX),通过将病人新鲜的瘤组织直接移植到免疫缺陷小鼠体内而建立的肿瘤模型,常见接种部位为皮下,原位。具体流程如下图。
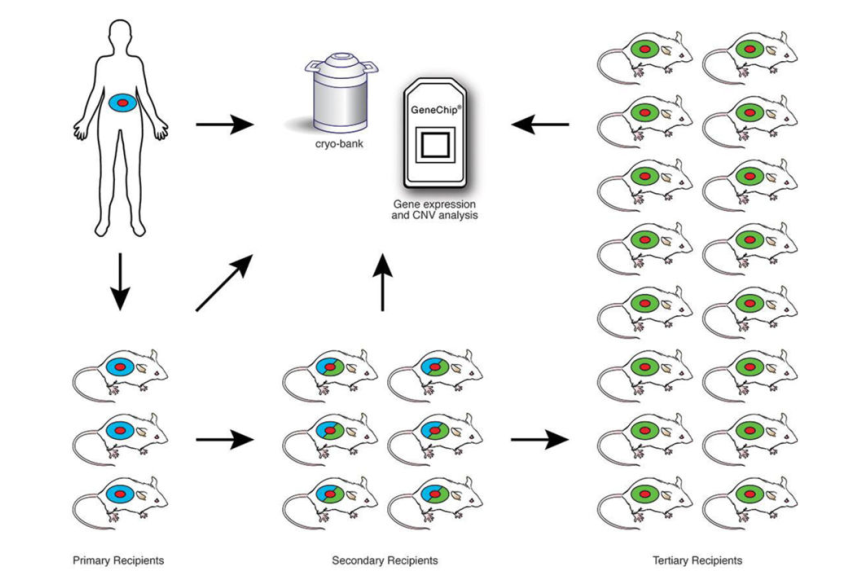
Fig. 1 Engraftment and expansion of patient-derived solid tumor xenografts. Young adult NSG
mice are engrafted with solid tumors (shown as red) Human stroma accompanying the tumor
is shown in blue. Primary solid tumors are engrafted subcutaneously, orthotopically, or
under the renal capsule. Established grafts are excised from primary recipients, a portion is
cryopreserved, and a portion is analyzed for gene expression, including copy number
variation (CNV). The remainder is expanded through serial transplantation in secondary and
tertiary NSG recipients to generate cohorts of sufficient size for therapeutic trials. At each
stage tumor samples are cryopreserved and gene expression analyzed to compare with the
primary tumor analyzed directly ex vivo from the patient. The primary recipients of solid
tumors retain human-derived stroma (blue). Tumors in secondary recipients contain a
mixture of human-derived and mouse-derived stroma (mixture of blue and green). Tumors
in tertiary recipients contain predominantly mouse stroma (green)
【Human Cancer Growth and Therapy In NOD/SCID/IL2Rγnull (NSG)
Mice 】
既然能使科研界沿用几十年的CDX被“退休”,那就必然代表新生力量PDX青出于蓝,目前该模型被研究者普遍认可的优势主要包括【人体肿瘤PDX移植模型的优与劣 】:
1. 移植所用标本直接来源于人体肿瘤组织,未经过体外培养,稳定地保留了肿瘤的遗传特性、组织学和表型特征,即肿瘤异质性;
如下图所示,为构建的人源结直肠癌小鼠移植模型,通过不同形态学特征的组织染色,发现在某些情况下,新鲜病灶和传代病灶均表现出良好的分化表型,某些样本均出现透明细胞,黏液分泌细胞和细胞坏死区域等,同时,少数肿瘤表现出高度的多形性,保持未分化状态(Fig2B)。
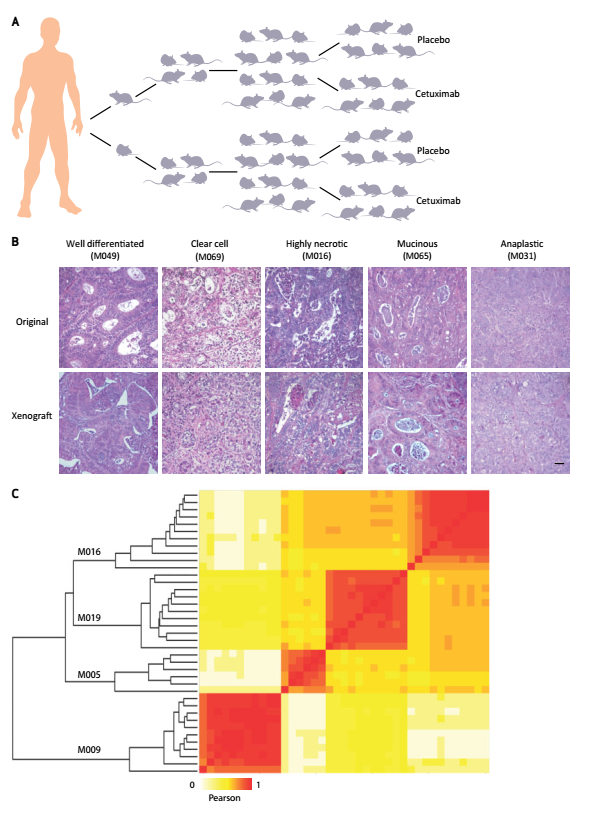
Figure 1. Setup and characterization of the xenopatient platform. A, generation of xenopatients. After surgical removal from patient, each metastatic colorectal cancer specimen was cut in small pieces and 2 fragments were implanted in 2 mice. After engraftment and tumor mass formation, the tumors were passaged and expanded for 2 generations until production of 2 cohorts, each consisting of 12 mice. These were randomized for treatment with placebo (6 mice) or cetuximab (6 mice). B, xenografted tumors retained the histopathologic characteristics of original samples. Hematoxylin and eosin stains of representative cases with different morphologic features. In some instances, both fresh and passaged lesions displayed a well-differentiated phenotype, with cells describing irregular pluristratified tubular/acinar structures with multiple lumens embedded in a scarce stromal matrix. Other samples had a clear-cell appearance or featured high nuclear grade and areas of necrosis. In some cases, discohesive mucus-secreting cells defined a moderately differentiated phenotype typical of mucinuos adenocarcinoma, with signet-ring elements showing peripheral nuclear delocalization and abundant intervening stroma associated with desmoplastic reaction. Finally, a few tumors exhibited high-grade pleomorphism and could be pathologically
classified as poorly differentiated adenocarcinomas. Scale bar, 50 μm. C, genetic concordance between xenografts and their original counterparts. Similar groups of samples are evidenced by applying a Pearson-based hierarchical clustering to copy number calls
2.PDX可用于筛选化疗药物敏感或耐药标记物,其试验结果具有较好的临床预见性
以胃癌PDX为例,图A表示对 cetuximab治疗不敏感与敏感小鼠中EGFR DNA/mRNA/EGFR受体蛋白拷贝数差异,发现敏感小鼠中拷贝数普遍较高,并且通过肿瘤生长曲线,IHC, FISH对比,最终得出:对 cetuximab敏感的小鼠模型,其EGFR表达普遍上调。
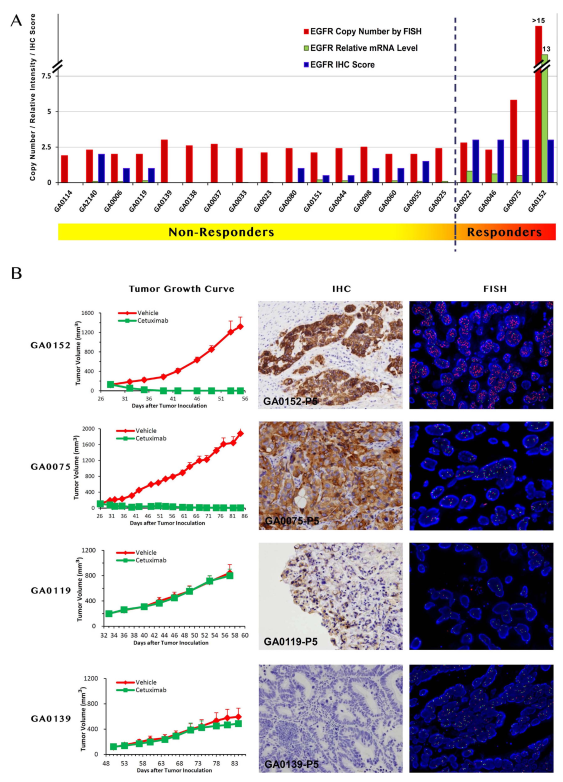
Figure 1 | The response to cetuximab treatment and genetic profile of GC-PDX models. Panel A: The PDX-GC models are sorted by the tumor response to cetuximab (DT/DC). The responders at the right part display higher EGFR mRNA level and IHC staining intensity, and the only two cases (GA0075 and GA0152, CN . 5) of gene amplification. Panel B: The representative images of responders and non-responders. The responders GA0152 and GA0075 display IHC score 31, and gene amplification (GA0075, CN 5 5.8; GA0152, CN . 15), while non-responders GA0119 and GA0139 are with IHC low
expression and no gene amplification. Left: Representative tumor growth curves of responders and non-responders. Middle: IHC analysis of tumor models; Right: Dual-color FISH assay in gastric carcinoma. Probe for EGFR locus is labeled in red and CEP7 labeled in green. Blue: Nuclei.
除此外,另有PDX在移植过程中较好地保留了肿瘤间质和干细胞成分,使得肿瘤的生长微环境更接近实际情况,还可为肿瘤样本的保存和传代提供大量标本等。
刚开始小编也说了,建模是为了什么?筛药!
这些荷瘤小鼠可以作为病人的“替身”(avatar),代替病人去测试不同药物方案的治疗效果,从中筛选出最有效的治疗方案,从而避免药物毒副作用以及经济上的浪费【PDX模型与肿瘤个体化用药指导 】,具体流程参照下图。

Establishment and testing of PDTX models. Excess tumour specimens not needed for clinical diagnosis are obtained from the consented patients (F0). Non-necrotic areas of these
tumours are sectioned into ~3 mm3 pieces and, after processing, implanted subcutaneously
into anaesthetized 5-week to 6-week-old female athymic nude mice. During the engraftment
phase, tumours are allowed to establish and grow and then are harvested upon reaching a
size of 1,500 mm3 (F1). Similar protocols are employed for subsequent expansion cohort
(F2) and treatment cohort (F3 … Fn). Typically, biological assays are performed on tumours
in early generations (≤F5); these biological assays include drug efficacy studies, rational
combination studies and the development of predictive biomarkers for novel targeted
therapies. If the developed biomarkers achieved accurate prediction in a validation set of
PDTX models (or ‘xenopatients’), they might be translated into early phase clinical trials as
tools for patient selection strategies. Abbreviations: PDTX, patient-derived tumour
xenografts; RES, resistant; SEN, sensitive. 【Patient-derived tumour xenografts as models for oncology drug development 】
当然了,虽然PDX优势颇多,但局限性也不容忽视【PDX 模型在肿瘤转化医学中的应用与发展 】
目前PDX模型原始肿瘤的主要来源为手术切除,建模难度高且不能反复获取,构建时间长且成功率不稳定,随着传代次数增加肿瘤微环境也会逐渐被小鼠细胞外基质取代,因此对于传代次数有一定限制。值得一提的是,负荷肿瘤的小鼠均为免疫缺陷的小鼠,因而该模型也无法用于筛选免疫相关药物。
总的来说,两种模型各有千秋,某种程度上CDX可以被认为是传代次数太多已经难以追溯的PDX模型,【PDX模型与肿瘤个体化用药指导 】经过体内外交替传代,原始肿瘤组织中只有最适应体外培养条件的单个克隆被保留下来,丧失了克隆异质性,从而难以对临床药效进行预测。
而PDX最大的优势也在于此,保留了肿瘤异质性,更符合临床肿瘤特征。
百奥赛图利用自主开发的重度免疫缺陷B-NDG小鼠,也建立了自己的PDX模型,由于所用小鼠全部为重度免疫缺陷小鼠,更好的保留了肿瘤组织的异质性,尤其对于普通免疫缺陷小鼠(裸鼠,NOD-scid小鼠)建模困难的血液瘤等PDX模型,有更好的重建成功率!对您的实验会有帮助吗?欢迎随时跟我们联系!
【参考文献】
[1] Cancer Cell Lines for Drug Discovery and Development
[2] PDX模型与肿瘤个体化用药指导
[3] Relationships between drug activity in NCI
